What is the total translational kinetic energy in a test chamber filled with nitrogen (N2) at 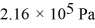 and
and 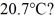 The dimensions of the chamber are
The dimensions of the chamber are 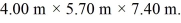 The ATOMIC weight of nitrogen is 28.0 g/mol, Avogadro's number is 6.022 × 1023 molecules/mol and the Boltzmann constant is 1.38 × 10-23 J/K.
The ATOMIC weight of nitrogen is 28.0 g/mol, Avogadro's number is 6.022 × 1023 molecules/mol and the Boltzmann constant is 1.38 × 10-23 J/K.
Correct Answer:
Verified
Q4: An ideal gas is kept in a
Q5: A mole of oxygen (O2)molecules and a
Q11: According to the second law of thermodynamics,
Q11: The second law of thermodynamics leads us
Q16: A 5.0-liter gas tank holds 1.4 moles
Q19: A hot piece of iron is thrown
Q19: If we double the root-mean-square speed (thermal
Q22: The root-mean-square speed (thermal speed)for a certain
Q23: An ideal gas is kept in a
Q27: A 5.0-liter gas tank holds 1.7 moles
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents