A 648-g empty iron kettle is put on a stove. How much heat. in joules. must it absorb to raise its temperature from 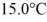 to
to 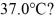 (The specific heat for iron is 113 cal/kg ∙ C°, 1 cal = 4.190 J)
(The specific heat for iron is 113 cal/kg ∙ C°, 1 cal = 4.190 J)
A) 6740 J
B) 11,300 J
C) 1610 J
D) 16,100 J
Correct Answer:
Verified
Q1: When an ideal gas increases in volume
Q3: A container of ideal gas has a
Q7: When a fixed amount of ideal gas
Q13: When a fixed amount of ideal gas
Q15: A thermally isolated system is made up
Q19: A chunk of ice (T = -20°C)is
Q20: The process shown in the pV diagram
Q22: If we use 67 W of power
Q29: It is necessary to determine the specific
Q33: A 905-g meteor impacts the earth at
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents