Heat is added to a 2.0 kg piece of ice at a rate of 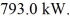 How long will it take for the ice to melt if it was initially at 0.00°C? (The latent heat of fusion for water is 334 kJ/kg and its latent heat of vaporization is 2260 kJ/kg.)
How long will it take for the ice to melt if it was initially at 0.00°C? (The latent heat of fusion for water is 334 kJ/kg and its latent heat of vaporization is 2260 kJ/kg.)
A) 0.84 s
B) 530,000 s
C) 4.7 s
D) 670 s
Correct Answer:
Verified
Q1: When an ideal gas increases in volume
Q20: The process shown in the pV diagram
Q22: What is the steady state rate of
Q26: How many grams of ice at -13°C
Q30: A block of ice at 0.000°C is
Q33: Two experimental runs are performed to determine
Q37: A person makes ice tea by adding
Q39: A substance has a melting point of
Q40: Two experimental runs are performed to determine
Q56: A copper cylinder with a mass of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents