The figure shows a pV diagram for 8.3 g of nitrogen gas (N2) in a sealed container. The temperature T1 of the gas in state 1 is 79°C. What are (a) the pressure p1 of the gas in state 1 and (b) the temperature T2 of the gas in state 2? The ideal gas constant is R = 8.314 J/mol ∙ K = 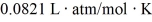 , and the ATOMIC weight of nitrogen is 14 g/mol.
, and the ATOMIC weight of nitrogen is 14 g/mol. 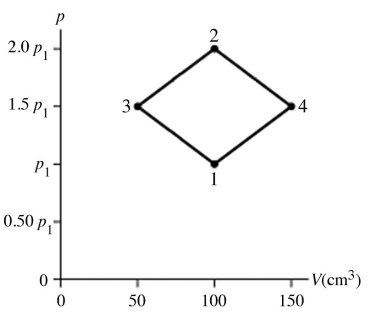
A) (a) 86 atm, (b) 700°C.
B) (a) 19 atm, (b) 700°C.
C) (a) 86 atm, (b) 160°C.
D) (a) 19 atm, (b) 160°C.
Correct Answer:
Verified
Q44: The walls of an ice chest are
Q46: A solid metal sphere is 15.0 cm
Q48: The figure (not to scale) shows a
Q48: A cube at 100.0°C radiates heat at
Q49: Some properties of glass are listed here.
Q51: A concrete wall of a cold storage
Q53: Betelgeuse is a red supergiant star in
Q54: A spherical object 25.0 cm in diameter
Q55: A rod, with sides insulated to prevent
Q58: The figure shows a pV diagram for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents