Which structure BEST describes the polarity of an H-O bond?
A) 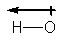
B) 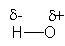
C) 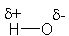
Correct Answer:
Verified
Q20: What is the electronic geometry around the
Q21: Which atom is the MOST electronegative?
A) Ca
B)
Q22: Which molecule will have the STRONGEST attraction
Q24: What is the correct ELECTRONIC GEOMETRY and
Q26: Based on the Pauling electronegativities,an Fr-F bond
Q27: Based on the Pauling electronegativities,a C-H bond
Q28: Which molecule has a NET DIPOLE?
A)
Q29: Based on the Pauling electronegativities,a P-Cl bond
Q30: Which atom is the MOST electronegative?
A) Li
B)
Q30: Which molecule(s) is/are likely to be a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents