Which of these symbols represents an atom with 15 protons, 16 neutrons, and 15 electrons?
A) 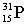
B) 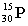
C) 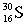
D) 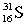
Correct Answer:
Verified
Q2: Which subatomic particle will be attracted to
Q7: Which subatomic particle will be attracted to
Q11: What two particles reside in the nucleus?
A)
Q14: The atomic number tells us:
A) the sum
Q17: Which subatomic particle has the LOWEST mass?
A)
Q21: Which symbol BEST represents a potassium cation?
A)
Q22: Which symbol represents an atom with 8
Q25: Which scientist proposed that electrons orbit the
Q26: Which symbol BEST represents a lithium cation?
A)
Q28: A certain element is composed of two
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents