The atomic symbol 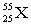 represents an element with _____ protons and _____ neutrons, respectively.
represents an element with _____ protons and _____ neutrons, respectively.
A) 25; 30
B) 25; 55
C) 55; 25
D) 55; 30
Correct Answer:
Verified
Q2: When an atom undergoes beta decay:
A) the
Q4: Which atomic symbol describes an atom with
Q4: Which factor determines the elemental identity of
Q5: Complete the following nuclear equation.
Q6: Which nuclide is represented by the atomic
Q10: Complete the following example of beta
Q11: Complete the following nuclear equation.
Q14: Isotopes,by definition,have the same _ but a
Q14: Complete the following nuclear equation.
Q19: The mass number is defined as the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents