Select the BEST description for these two molecules. 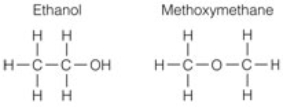
A) They are isomers because they have the same formula but different structures.
B) They are isomers because they have the same number of atoms.
C) They are not isomers because the oxygen is connected differently.
D) They are isomers because they have the same formula and both have all single bonds.
Correct Answer:
Verified
Q1: Isomers are defined as:
A) lines on a
Q2: How many structural isomers are possible for
Q3: How many structural isomers are possible for
Q4: How many structural isomers are possible for
Q5: Draw a proper Lewis structure for C2H6.The
Q6: The two products of hydrocarbon combustion are:
A)
Q14: How many structural isomers are possible for
Q17: Draw a proper Lewis structure for HCN.The
Q19: In forming molecular compounds,the nonmetals carbon,nitrogen,oxygen,and fluorine
Q20: Draw a proper Lewis structure for C2H2.The
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents