If the accompanying drawing of an electrochemical cell represents an iron-nickel cell, use the partial activity series included to determine the component labeled 1. 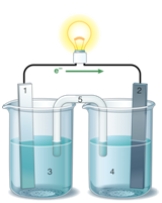 Zn Zn2+ + 2e- Fe Fe2+ + 2e-
Zn Zn2+ + 2e- Fe Fe2+ + 2e-
Co Co2+ + 2e-
Ni Ni2+ + 2e-
Cu Cu2+ + 2e-
A) iron anode
B) nickel anode
C) iron cathode
D) nickel cathode
Correct Answer:
Verified
Q22: Given the accompanying partial activity series,which
Q35: The oxidation half-reaction for the reaction
Q35: If the accompanying drawing of an
Q37: If the accompanying drawing of an
Q39: Knowing that sodium and potassium react violently
Q43: Write the balanced net ionic equation
Q44: Use the partial activity series included
Q46: Which reaction could be used to
Q47: Which pair of half-reactions takes place
Q51: Write the balanced net ionic equation
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents