The figure shows a pV diagram for a gas going through a cycle from A to B to C and back to A. From point A to point B, the gas absorbs 50 J of heat and finds its internal (thermal)energy has increased by 20 J. Going from B to C, the internal (thermal)energy decreases by 5.0 J.
(a)How much work was done by the gas from A to B?
(b)How much heat was absorbed by the gas from B to C?
(c)How much work was done by the gas going from B to C? 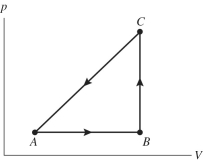
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q21: In an isochoric process,the internal (thermal)energy of
Q25: In an adiabatic compression,200 J of work
Q27: Platinum melts at 3215°F.What is the corresponding
Q29: A heat engine absorbs 64 kcal of
Q30: A person consumes a snack containing 14
Q38: An ideal gas undergoes an adiabatic process
Q41: A cylinder contains 8.8 moles of
Q43: An ideal gas undergoes the process a→b→c→a
Q49: A cylinder contains 10 moles of an
Q59: A cylinder contains 1.50 moles of an
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents