For the image shown below, choose which line represents the gas at the lowest temperature.The x-axis represents the molecular speed in m/s and the y-axis represents the number of atoms. 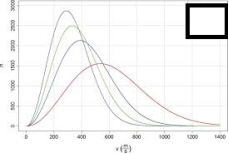
A) The tallest, narrowest line
B) The shortest, widest line
C) The second tallest, second narrowest line
D) The third tallest, third narrowest line
Correct Answer:
Verified
Q32: Which of the following gases would move
Q33: Which describes an experiment in which only
Q34: If a sealed box at STP holds
Q35: At what temperature is a gas considered
Q36: A rigid, sealed box at 25°C is
Q37: A balloon at STP holds 2.4 ×
Q38: Of the four gas variables, which are
Q39: Another name for hydraulic fracturing, the process
Q40: Which describes an experiment in which only
Q42: For the image shown below, choose which
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents