Identical heat lamps are arranged to shine on two identical containers, one containing water and one methanol (wood alcohol) , so that each liquid absorbs the same amount of energy minute by minute. The covalent bonds of methanol molecules are nonpolar, so there are no hydrogen bonds among methanol molecules. Which of the following graphs correctly describes what will happen to the temperature of the water and the methanol?
A) 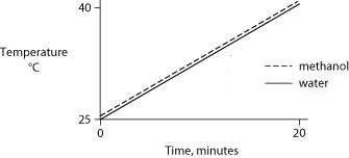
B) 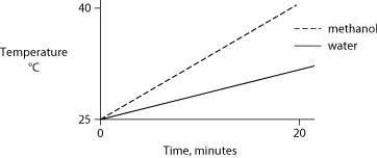
C) 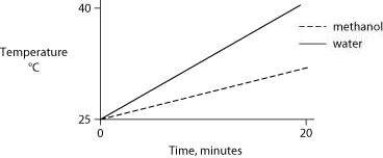
D) 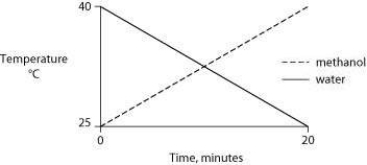
Correct Answer:
Verified
Q26: What is the pH of a solution
Q27: A solution contains 0.0000001 (10⁻⁷) moles of
Q28: Consider the following reaction at equilibrium: CO₂
Q29: How many grams of the compound in
Q30: Which of the following is considered to
Q32: A 0.01 M solution of a substance
Q33: How much of 0.5 M glucose (molecular
Q35: A solution with a pH of 2
Q36: Use the following figure to answer the
Q38: Rank, from low to high, the pH
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents