Which cell diagram is correct for this electrochemical cell? 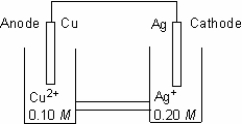
A) Ag(s) |Ag+(aq) (0.20 M) ||Cu(s) |Cu2+(aq) (0.10 M)
B) Ag(s) |Cu2+(aq) (0.10 M) ||Ag+(aq) (0.20 M) |Cu(s)
C) Ag+(aq) (0.20 M) |Ag(s) ||Cu2+(aq) (0.10 M) |Cu(s)
D) Cu(s) |Cu2+(aq) (0.10 M) ||Ag+(aq) (0.20 M) |Ag(s)
E) Cu(s) |Cu2+(aq) (0.10 M) ||Ag(s) | Ag+(aq) (0.20 M)
Correct Answer:
Verified
Q28: A voltaic cell is constructed based on
Q29: Consider the following standard reduction potentials.
Q30: Which statement about a voltaic cell is
Q32: Proteins containing a certain functional group
Q34: The electrodes on batteries are labeled +
Q35: This is a true story; can
Q36: Which statement is not correct for a
Q36: The electrodes on batteries are labeled +
Q37: Which statement about a cathode in a
Q38: Consider an electrochemical cell with a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents