The following figures represent distributions of two types of gas molecules between two containers connected by an open tube. In which figure is the entropy of the system maximized?
A) 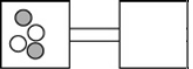
B) 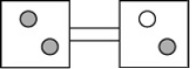
C) 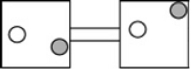
D) 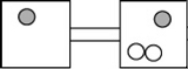
Correct Answer:
Verified
Q22: Determine
Q23: Determine
Q24: Determine the entropy change for the
Q25: If 3.500 g of Ni are
Q26: The standard molar entropy of lead(II) bromide
Q28: Perfect crystals of carbon monoxide (CO) are
Q29: Which of the following is in the
Q30: The following figures represent distributions of gas
Q31: At 0 K, the entropy of a
Q32: Which of the following will have the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents