A sketch of the free energy for a hypothetical chemical equilibrium is shown here. What part of the plot on the axis representing the relative quantities of reactants and products corresponds to a value of Q that is less than K? 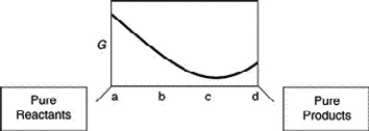
A) a to b
B) b to c
C) a to c
D) b to d
E) c to d
Correct Answer:
Verified
Q113: As T approaches infinity, ln K
Q115: What is the overall standard free-energy
Q116: What is the overall standard free-energy
Q119: The equilibrium constant for a reaction
Q120: A qualitative interpretation of the effect
Q121: The standard molar enthalpy of fusion for
Q132: The equilibrium constant for a given reaction
Q145: An oxygen molecule can have several different
Q146: Of the three modes of molecular motion-vibration,
Q179: Name and state the first three laws
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents