What is indicated by the shape of the titration curve? 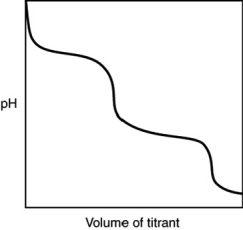
A) A diprotic acid was titrated with a strong base.
B) A triprotic acid was titrated with a strong base.
C) A diprotic base was titrated with a strong acid.
D) A triprotic base was titrated with a strong acid.
E) A strong acid was titrated with a strong base.
Correct Answer:
Verified
Q10: Which of the following can be mixed
Q15: Suppression of the solubility of one ion
Q29: Glycolic acid, which is a monoprotic acid
Q30: Lactic acid, which is found in milk
Q39: What are the characteristics of a pH
Q44: A solution of sulfuric acid (H2SO4, 25.00
Q49: A solution of hydrochloric acid (HCl, 25.00
Q125: The following titration curve is most likely
Q126: You have a summer job as an
Q127: A phosphate buffer solution (25.00 mL sample)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents