A 0.500 g sample of an unknown substance was titrated with a 0.1 M HCl solution. Another 0.500 g sample of it was titrated with a 0.1 M NaOH solution. The resulting titration curves are illustrated here. Given the following possibilities, what is the sample? 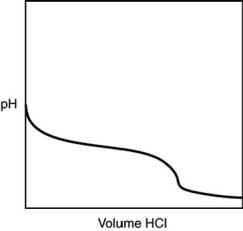
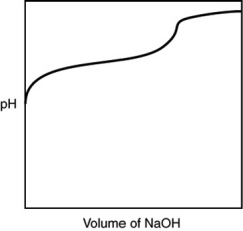
A) Na2CO3
B) NaHCO3
C) H2CO3
D) CO2
E) There is no way to tell.
Correct Answer:
Verified
Q45: A phosphate buffer solution (25.00 mL sample)
Q46: One brand of extra-strength antacid tablets contains
Q54: Vitamin C is a monoprotic weak acid,
Q108: Which sodium halide salt, if any, would
Q133: Halfway to the equivalence point in a
Q135: Stalactites-the long, icicle-like formations that hang from
Q136: The following titration curve is most likely
Q139: In a titration of monoprotic acids and
Q142: Stalactites-the long, icicle-like formations that hang from
Q143: A mining operation needs to separate silver
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents