Indicate which of the following plots would be obtained for a first-order reaction.
A) 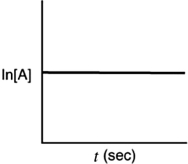
B) 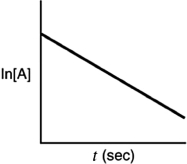
C) 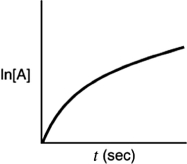
D) 
Correct Answer:
Verified
Q63: The linear form of the Arrhenius equation
Q73: Collision theory assumes that the rate of
Q75: Given the following data, determine the
Q76: Which of the following plots would indicate
Q77: Given the following data, determine the
Q79: Which of the following plots indicates that
Q81: The energy profiles for four different reactions
Q82: The energy profiles for four different reactions
Q84: Which point as labeled by an asterisk
Q85: The linear form of the Arrhenius equation
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents