A molecule with the formula C7H14 was found to have carbons that were all identical in terms of structure. That is, each carbon atom was bonded to exactly the same kinds of atoms as all the rest. What is a reasonable structure for this molecule?
A) CH3(CH2) 5CH3
B) 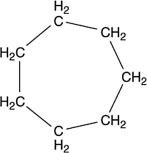
C) CH2(CH2) 5CH2
D) 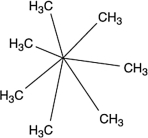
Correct Answer:
Verified
Q27: Identify the molecules that are structural isomers
Q28: What is the molecular formula for the
Q29: Which of the following is/are true regarding
Q30: Which statement best describes how these three
Q31: The C-C-C bond angles in octane (CH3CH2CH2CH2CH2CH2CH2CH3)
Q33: Name the compound with the structural formula
Q34: What is the molecular formula for the
Q35: Which of the following pairs has the
Q36: The -CH2- unit is known as _
A)
Q37: Which of the following is a methyl
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents