Which one of the following substances would you predict to have the highest vapor pressure at a given temperature? In these line drawings, a carbon is implicit at the end of a line (bond) or where two or more lines come together. Carbon requires four bonds, so any missing bonds are implicitly bonds to hydrogen atoms.
A) 
B) 
C) 
D) 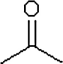
E) 
Correct Answer:
Verified
Q46: Which of the following compounds would be
Q48: Which of the following compounds do you
Q51: The aroma from almonds and cherries is
Q52: Which alcohol should be most soluble in
Q56: Which of the following pairs of compounds
Q56: Gasoline is primarily a mixture of hydrocarbons
Q57: Portable lanterns and stoves used for camping
Q61: Which alkane compound has the highest boiling
Q66: Which of the following statements does not
Q76: The vapor pressure of a liquid will
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents