The phase diagram for carbon dioxide is shown below. What is the phase that exists at room temperature and pressure (22oC, 1 atm) ? 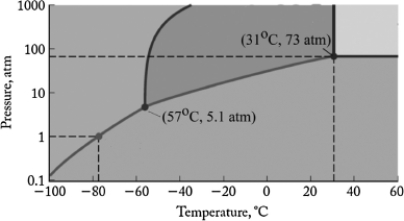
A) gas
B) liquid
C) solid I
D) supercritical fluid
E) solid II
Correct Answer:
Verified
Q75: Sublimation occurs in going from region _
Q76: Melting occurs in going from region _
Q77: What does the line indicated by the
Q79: Point d in the phase diagram below
Q81: Carbon dioxide is being used as an
Q83: Which is the dominant interaction between acetone
Q84: Which is the dominant interaction between carbon
Q86: At the critical point, _
A)all the liquid
Q108: Which is the dominant interaction between water
Q118: Water forms a concave meniscus in a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents