The phase diagram for carbon dioxide is shown below. What are the phase changes in order as the pressure is increased starting at 0.5 atm, and the temperature is kept at -80oC? 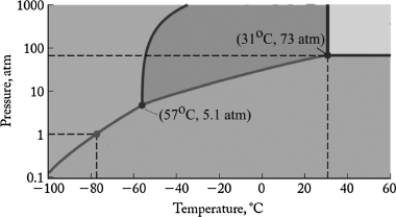
A) solid
Gas
B) solid
Liquid
Gas
C) liquid
Gas
D) gas
Solid
E) gas
Liquid
Correct Answer:
Verified
Q93: Which hydride do you predict has the
Q94: The phase diagram for carbon dioxide is
Q98: The density of water decreases as it
Q99: The phase diagram for carbon dioxide
Q100: The phase diagram for carbon dioxide is
Q110: The resistance of a liquid to an
Q111: Which type of intermolecular interaction exists for
Q112: Which is the dominant interaction that leads
Q129: Which of the following compounds would you
Q139: Which of the following gases would you
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents