Which one of the following substances would you predict to have the highest boiling point? In these line drawings, a carbon is implicit at the end of a line (bond) or where two or more lines come together. Carbon requires four bonds, so any missing bonds are implicitly bonds to hydrogen atoms.
A) 
B) 
C) 
D) 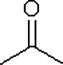
E) 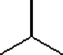
Correct Answer:
Verified
Q105: In understanding why ammonia (NH3) has a
Q106: CH2F2 has a dipole moment of 1.93
Q108: Given the van der Waals a constant
Q109: Given the van der Waals a constant
Q112: The relative energies (strengths) of the intermolecular
Q113: In understanding why group 16 hydrides, other
Q114: The relative energies (strengths) of the intermolecular
Q115: The relative energies (strengths) of the intermolecular
Q124: Which of the following substances would you
Q127: The boiling point of HBr is higher
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents