Which of the following represents the best Lewis structure for dinitrogen sulfide?
A) 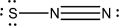
B) 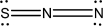
C) 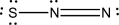
D) 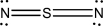
E) 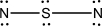
Correct Answer:
Verified
Q91: What is the formal charge of the
Q92: Which structure for dinitrogen sulfide (N _N
Q93: The formal charge on the oxygen atoms
Q97: Use the formal charge as a criterion
Q99: What is the formal charge on the
Q100: Odd electron molecules are called _
A) anions.
B)
Q102: How many lone-pair electrons are on the
Q114: In which of the following molecules does
Q115: Which of the following molecules contains an
Q117: Which of the following atoms can have
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents