Polymers with ionic functional groups have been developed for removal of ions from water. One example is Amberlite. One form of Amberlite has the structure shown below, where the -SO3H groups act like a weak acid. What type of ions will this polymer attract? 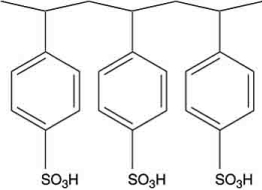
A) cations
B) anions
C) all ions
D) impossible to tell
E) none, since the polymer is neutral
Correct Answer:
Verified
Q79: Sodium thiosulfate (Na2S2O3, molar mass =
Q80: A reaction that takes place between
Q81: Chlorine, ozone, and chlorine dioxide are all
Q83: Groundwater often has low levels of
Q87: In which compound does chlorine have an
Q88: Methane (CH4) is a suitable fuel for
Q89: Which one of the following statements is
Q89: The thermite reaction is used in
Q98: Sodium metal easily loses an electron to
Q104: Lead sinkers used for saltwater fishing do
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents