Provide a neatly drawn mechanism for the following reaction, including curved arrows to show the movement of paris of electrons and the structure of reactive intermediates. 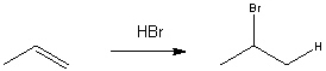 The reaction proceeds in two steps:
The reaction proceeds in two steps:
1. Protonation of propene to form the 2-propyl cation
2. Nucleophilic addition of bromide anion to the 2-propyl cation
Correct Answer:
Verified
Q23: What type of reaction mechanism accounts for
Q26: What type of reaction mechanism accounts for
Q29: What type of reactive intermediate is formed
Q59: Which of the following reactions of alkenes
Q61: What is the best choice of reagent(s)
Q63: What is the best choice of reagent(s)
Q64: Provide a neatly drawn mechanism for the
Q65: What is the best choice of reagent(s)
Q66: Provide the structure of the key intermediate
Q67: What is the major organic product obtained
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents