Complete the equation below for the protonation of cyclohexene with HCl. Show the movement of pairs of electrons with curved arrows and provide the structures of the conjugate acid and conjugate base.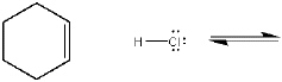
Correct Answer:
Verified
Q63: Provide a brief explanation, based on features
Q64: What is the value of the equilibrium
Q65: Provide a brief explanation, based on features
Q66: Provide the equation for the acid dissociate
Q68: What is the value of the equilibrium
Q69: What is the value of the equilibrium
Q70: Use curved arrows to show the movement
Q71: Provide the equation that relates the equilibrium
Q72: Use curved arrows to show the movement
Q75: Provide the equation that relates the acid
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents