Which of the following best represents an sp3 hybridized atomic orbital containing the lone pair of electrons of ammonia, NH3? 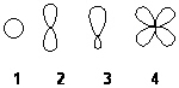
A) 1
B) 2
C) 3
D) 4
Correct Answer:
Verified
Q42: What is the approximate value of the
Q43: How many electrons are there in the
Q55: How many electrons are there in the
Q56: How many electrons are there in the
Q58: Which of the following statements is not
Q60: Which of the following best represents the
Q61: Which atomic orbitals overlap to form the
Q62: Which of the circled bonds is the
Q63: Which of the following shows curved arrows
Q64: Which of the following is a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents