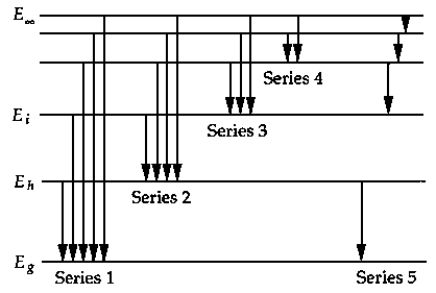
 The above figure shows a schematic energy-level diagram for the hydrogen atom. The series that represents the Balmer series is
The above figure shows a schematic energy-level diagram for the hydrogen atom. The series that represents the Balmer series is
A) 1.
B) 2.
C) 3.
D) 4.
E) 5.
Correct Answer:
Verified
Q11: The critical experiments that established the nuclear
Q12: The wavelength of the visible line in
Q20: The wavelength of the visible line in
Q39: What is the difference in wavelength between
Q72: An alpha particle (mass = 4 amu)is
Q85: A classical particle
A)exhibits interference.
B)exhibits diffraction.
C)has energy associated
Q88: Large neutron-scattering facilities have been established to
Q95: What is the energy difference between the
Q96: Calculate the wavelength of light of energy
Q97: Rutherford's experiments, in which he bombarded a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents