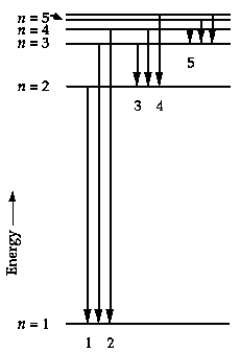
 In the energy-level diagram, the line that corresponds to the longest wavelength in the Balmer series is
In the energy-level diagram, the line that corresponds to the longest wavelength in the Balmer series is
A) 1.
B) 2.
C) 3.
D) 4.
E) 5.
Correct Answer:
Verified
Q12: The wavelength of the visible line in
Q20: The wavelength of the visible line in
Q39: What is the difference in wavelength between
Q83: A classical wave
A)behaves like a water wave.
B)exhibits
Q83: What is the energy difference between the
Q84: For an x-ray tube to produce x
Q85: The constant in the Rydberg formula is
Q85: A classical particle
A)exhibits interference.
B)exhibits diffraction.
C)has energy associated
Q88: Large neutron-scattering facilities have been established to
Q94: Which of the following statements is/are true?
A)Nothing
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents