The energy of the nth level in a one-electron atom is 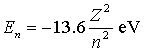 . Consider a beryllium ion with all but one of its electrons removed a beryllium atom normally has four electrons) . What is the wavelength of a photon emitted when the electron makes the transition from the third-lowest to the lowest energy state?
. Consider a beryllium ion with all but one of its electrons removed a beryllium atom normally has four electrons) . What is the wavelength of a photon emitted when the electron makes the transition from the third-lowest to the lowest energy state?
A) 1.03 × 10-7 m
B) 2.03 × 10-8 m
C) 6.43 × 10-9 m
D) 5.71 × 10-9 m
E) 1.03 × 10-27 m
Correct Answer:
Verified
Q26: A photon of wavelength 80 nm is
Q29: The red line in the hydrogen emission
Q34: An electron in a hydrogen atom jumps
Q122: What is the magnitude of the change
Q123: The electron in a hydrogen atom has
Q124: Light of wavelength 411 nm is observed
Q125: According to Bohr's model, the radius of
Q127: 6 eV. The binding energy of the
Q129: According to the Bohr theory, the allowed
Q130: In the Bohr model of the hydrogen
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents