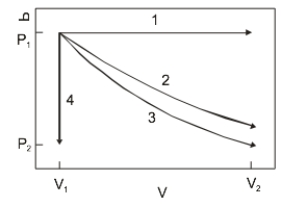
 The diagram above show the state of an ideal gas going from V1, P1) to a final state. Which path best represents an isothermal expansion?
The diagram above show the state of an ideal gas going from V1, P1) to a final state. Which path best represents an isothermal expansion?
A) 1
B) 2
C) 3
D) 4
E) None of the paths.
Correct Answer:
Verified
Q34: The specific heat of a gas is
A)the
Q39: Q40: The equation of state for a certain Q42: The internal energy for a diatomic gas Q45: One mole of an ideal gas γ Q46: For an ideal gas, the difference in Q47: The pressure of a mass of air Q48: A gas has a molar heat capacity Q49: One mole of an ideal gas γ Q91: A gas can absorb heat without changing![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents