In the acid-base reaction between ammonia and water, which of the following statements best describes the concentration of ammonia and ammonium at equilibrium? 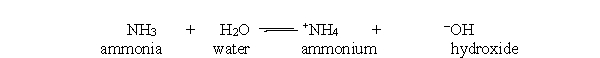
A) The concentration of ammonium is increasing.
B) There is no ammonium at equilibrium.
C) Their concentrations are equal.
D) Their concentrations are constant.
E) There is no ammonia at equilibrium.
Correct Answer:
Verified
Q10: Generally, strong bases are hydroxide salts of
A)
Q18: A conjugate acid-base pair is
A) the reactants
Q38: Each circle is a sample of an
Q40: Which of the following acids is not
Q41: An alkene has a pKa of 40,
Q43: In the acid-base reaction between ammonia and
Q44: The pKa of aspirin is 3.5. What
Q45: Arsenic poisoning is a serious problem in
Q46: Methylamide (CH3CONH2) has a Ka of 1
Q47: Which substance is acting as the acid
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents