A buffer solution contains H2CO3 and HCO3 - , resulting in the equilibrium shown by the reaction below. According to this reaction and LeChatelier's principle, what happens when acid (H3O+) is added to this buffer solution? 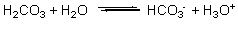
A) More HCO3 - is formed.
B) More H3O+ is formed.
C) More H2CO3 is formed
D) Less H2O is formed.
E) The reaction shifts to the right.
Correct Answer:
Verified
Q50: The neutralization reaction of potassium hydrogen carbonate
Q87: Which of the following is a balanced
Q88: The following figure illustrates the action of
Q89: The neutralization reaction of potassium hydrogen carbonate
Q90: Which of the following statements best describe
Q91: The following figure illustrates the action of
Q94: Lidocaine, a common injectable dental anesthetic, is
Q95: According to the chart below, how are
Q96: Metabolic acidosis is a type of acid-base
Q97: Gastric juice is the most acidic fluid
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents