The following figure illustrates the action of a HF and F - buffer where the sizes of the boxes are proportional to the concentrations of HF and F - in solution. If you add hydronium until all of the F - is converted into HF and then add a little more hydronium, what is observed? 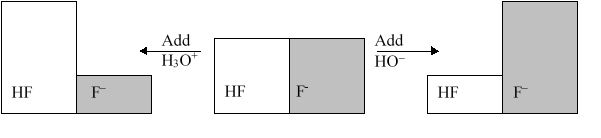
A) The pH increases.
B) The pH decreases.
C) The pH stays the same.
D) The solution will be neutralized.
E) The pH changes, but it is not possible to determine how it will change.
Correct Answer:
Verified
Q6: Which statement about neutralization reactions is FALSE?
A)
Q80: A sample of gastric juice has a
Q81: Which species in the following neutralization reaction
Q82: The following figure illustrates the action of
Q83: Lidocaine, a common injectable dental anesthetic, is
Q84: Which of the following is a buffer
Q87: Which of the following is a balanced
Q88: The following figure illustrates the action of
Q89: The neutralization reaction of potassium hydrogen carbonate
Q90: Which of the following statements best describe
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents