When a person hyperventilates, they breathe very quickly, exhaling CO2 more quickly than it can be produced. This reduces the concentration of CO2 in the blood. According to the chemical equation below and LeChatelier's principle, how is the bicarbonate equilibrium affected by the reduction of CO2 in the blood? 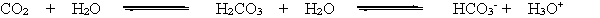
A) The equilibrium is unchanged.
B) More H3O+ is produced.
C) The equilibrium is shifted to the right.
D) The H3O+ concentration decreases.
E) More HCO3 - is produced.
Correct Answer:
Verified
Q47: What happens to pH when the buffer
Q102: Which of the following statements best describes
Q103: Which of the following buffer systems is
Q104: Which of the following changes in blood
Q105: What is an enteric coating?
A) It is
Q106: Where does digestion of proteins begin?
A) in
Q107: Which of the following would be the
Q109: How do Type I and Type II
Q110: Which of the following conditions could cause
Q111: The buffering system of the blood is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents