Consider the two containers below, which are separated by a semipermeable membrane that only allows the passage of small solutes. If A contains 6% NaCl in water and B contains 3% NaCl in water, which of the following will occur? 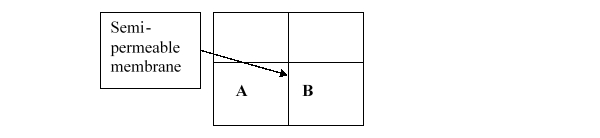
A) The volume of water in A will increase.
B) The volume of water in B will increase.
C) NaCl will flow from A to B.
D) NaCl will flow from B to A.
E) Nothing will happen.
Correct Answer:
Verified
Q24: A person receiving IV fluids must always
Q78: The cell membrane separates the interior of
Q79: Convert 0.015 M Ca2+ to equivalents of
Q80: How many moles of glucose are in
Q81: This substance in the blood regulates the
Q82: An abnormal concentration of this substance in
Q84: Consider solutions A and B separated by
Q85: Which term best describes the process illustrated
Q87: Red blood cells are placed into three
Q88: Consider solutions A and B separated by
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents