Does isopropyl alcohol have a higher or lower boiling point than acetone? 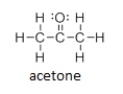
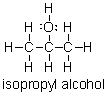
A) Higher, because isopropyl alcohol can hydrogen bond
B) Lower, because isopropyl alcohol can hydrogen bond
C) Higher, because isopropyl alcohol can form dispersion forces
D) Lower, because isopropyl alcohol can form dispersion forces
E) It is not possible to determine the answer based on the information provided.
Correct Answer:
Verified
Q33: The simplest aldehyde is formaldehyde, which is
Q48: What is the molecular shape of the
Q49: When administered as a treatment for anaphylaxis,
Q50: What is the IUPAC name of the
Q51: Which statement best describes why amine I
Q52: What is the name of the following
Q54: Can adrenaline form hydrogen bonds, and if
Q55: Which statement best describes how aldehydes, ketones
Q56: Which of the following illustrations best describes
Q57: Which of the following molecules contains at
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents