A common misconception is that the following molecule contains two functional groups, an alcohol and a ketone, but this is not the case. What functional group does this molecule actually contain and why is it important to differentiate between this functional group and an alcohol? 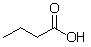
A) This molecule contains a carboxylic acid, which reacts very differently than an alcohol.
B) This molecule contains a carboxylic acid, which is an isomer of an alcohol.
C) This molecule contains a carbonyl, which hydrogen bonds much more than an alcohol.
D) This molecule contains a carbonyl, which reacts very differently than an alcohol.
E) This molecule contains a carboxylic acid, which is unreactive.
Correct Answer:
Verified
Q64: Which of the following molecules is ethyl
Q65: What is the name of the following
Q66: Which of the following molecules contain an
Q67: What type of biomolecule is shown below?
Q68: Methadone, a drug used to aid in
Q70: Which of the following biomolecules contains three
Q71: Which of the following molecules is acetic
Q72: What type of biomolecule is shown below?
Q73: Which of the following molecules has the
Q74: Which functional groups are in the following
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents