The following structure is a poor representation of benzene. What is wrong with it? 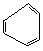
A) Benzene does not have six sides.
B) Benzene is not flat.
C) Benzene is never represented with three double bonds and three single bonds.
D) Benzene does not have the molecular formula C6H6.
E) The bonds in benzene should all be the same length.
Correct Answer:
Verified
Q78: What are the locator number(s) and substituent
Q79: What is the name of the following
Q80: Which of the following pairs of compounds
Q81: Benzene is not classified as an
Q82: What is the IUPAC name of this
Q84: A cyclohexane molecule and a benzene molecule
Q85: Which of the following structure(s) is represented
Q86: Aspirin contains a benzene ring substituted on
Q87: Electrons in benzene are spread out over
Q88: What is the IUPAC name of the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents