When heat energy is added to a solid and liquid mix at the melting point, the temperature does not increase, as illustrated by horizontal line C. Which of the statements below best describes what happens to the heat energy added to the solid and liquid? 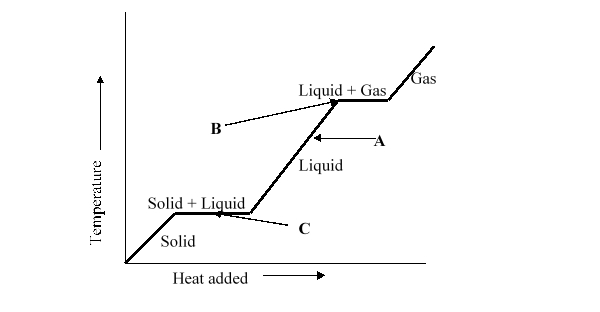
A) It increases the kinetic energy of the molecules.
B) It decreases the kinetic energy of the molecules.
C) It breaks the intermolecular forces between the molecules.
D) It makes new intermolecular forces between molecules.
E) It just passes through the solid and liquid, which is why the temperature does not increase.
Correct Answer:
Verified
Q8: In which of the following phase changes
Q9: Which process requires more energy per
Q10: Which statement best describes how heat energy
Q11: Why is water (H2O) a liquid at
Q15: Formaldehyde, a common preservative, is shown in
Q16: A liquid has temperature A as shown
Q17: How are vaporization and evaporation similar?
A) Both
Q18: What change of phase is represented by
Q62: How do phase changes differ from chemical
Q81: Which of the following changes of state
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents