Imagine that you have a beaker of gas molecules. A small volume of the gas in the beaker is enlarged so you can see the gas particles. If all of the gas in the beaker A is transferred to a new beaker half the size of the original beaker, while maintaining the same temperature, which magnified view best represents what the gas would look like? 
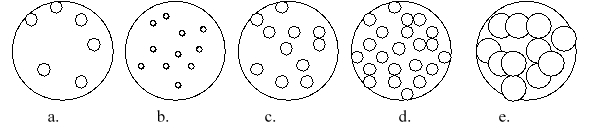
A) a
B) b
C) c
D) d
E) e
Correct Answer:
Verified
Q72: What happens when a large volume of
Q73: Which statement best describes how the kinetic
Q74: Which of the following statements best describes
Q75: Which of the following are ways to
Q76: You drive your car from Salt
Q78: Three boxes, each containing molecules in the
Q79: Which of the following is the molar
Q82: Which of the following statements best describes
Q90: Hyperbaric oxygen therapy is the use of
Q98: An oxygen tank has a pressure of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents