Which statement best describes how the temperature of the surroundings change as a result of the reaction represented by energy diagram below? 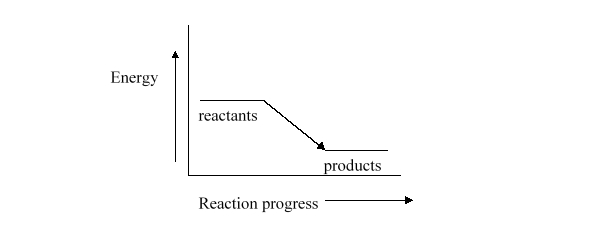
A) The temperature of the surroundings does not change.
B) The temperature of the surroundings increases.
C) The temperature of the surroundings decreases.
D) The surroundings will become very cold.
E) It is not possible to predict anything about the temperature of the surroundings based on this diagram.
Correct Answer:
Verified
Q6: A calorimeter is used to measure the
Q58: When bonds break, energy is
A) always released.
B)
Q75: How is the reaction that occurs in
Q88: Which of the following diagrams illustrates an
Q89: An exothermic reaction is one that
A) has
Q90: The food that we eat is composed
Q94: Which of the following reactions releases the
Q95: Which bonds are broken over the course
Q96: An endothermic reaction is one that
A) releases
Q97: Which of the following reactions could be
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents