The reaction between acetic acid (CH3COOH) and methanol (CH3OH) is given below, followed by a list of changes that could be made to the reaction. Which of these changes will result in the equilibrium shifting to the left? 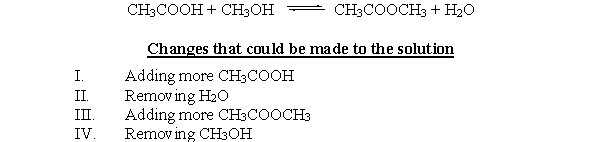
A) All of these changes will result in the equilibrium shifting to the left.
B) Only I will result in the equilibrium shifting to the left.
C) Only IV will result in the equilibrium shifting to the left.
D) I and III will result in the equilibrium shifting to the left.
E) III and IV will result in the equilibrium shifting to the left.
Correct Answer:
Verified
Q27: A spirometer is used in indirect calorimetry.What
Q28: An endothermic reaction absorbs heat when it
Q40: Which of the following statements describe how
Q125: Arsenic poisoning is a serious problem in
Q126: Which of the following statements best describes
Q128: What type of calorimetry is the most
Q131: The reaction of water with ammonia is
Q132: The reaction of water with ammonia is
Q133: Arsenic poisoning is a serious problem in
Q134: How do direct and indirect calorimetry differ?
A)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents