In this covalent molecule of methane, 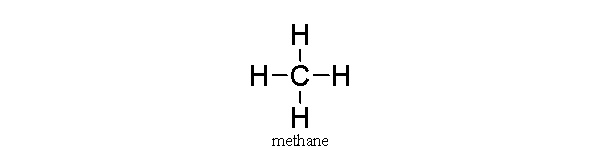
A) carbon is sharing its four electrons with the hydrogens.
B) carbon has donated four electrons to hydrogen, leaving it with a charge of +4.
C) hydrogen has eight electrons in its valence shell.
D) carbon is very unstable.
E) carbon does not have an octet of electrons.
Correct Answer:
Verified
Q25: In a covalent molecule
A) nonmetals share electrons.
B)
Q30: What is the ratio of ions in
Q32: What are the charges on the ions
Q33: Vinegar (C2H4O2) and baking soda (NaHCO3) are
Q37: Below is an illustration of an atom
Q39: Which of the following is a diatomic
Q40: Below are four molecules and compounds. Choose
Q46: What is the name of Na2SO3, a
Q49: Another name for an ionic compound is
Q50: A compound contains magnesium and phosphate.What is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents