Which of the structures are covalent molecules? 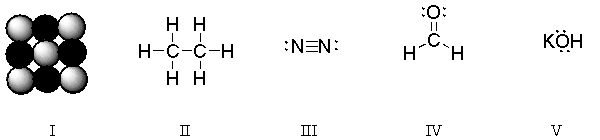
A) I and V
B) II, III, and IV
C) II and IV
D) V only
E) All of the choices are covalent molecules.
Correct Answer:
Verified
Q37: Below is an illustration of an atom
Q39: Which of the following is a diatomic
Q40: Below are four molecules and compounds. Choose
Q42: Which of the following statements best describes
Q43: How many of chlorine's electrons are unpaired?
A)
Q44: Which of these structures has two nonbonding
Q45: The total number of valence electrons in
Q46: The arrow is pointing to a(n)
A)
Q49: Another name for an ionic compound is
Q50: A compound contains magnesium and phosphate.What is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents