The structure below is the Lewis structure of methane. The Lewis structure tells us many things about methane that are useful. However, it also suggests one characteristic of methane that is, in fact, false. What is this one characteristic? 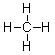
A) The Lewis structure suggests that carbon has a full valence shell, but it really doesn't.
B) The Lewis structure suggests that carbon has four bonds, but it really doesn't.
C) The Lewis structure suggests that carbon is bonded to four hydrogens, but it really isn't.
D) The Lewis structure suggests that methane is flat, but it really isn't.
E) The Lewis structure suggests that methane is stable, but it really isn't.
Correct Answer:
Verified
Q70: Why do electrons around a central atom
Q71: Which of the following elements might have
Q72: What is the meaning of the "wedge
Q73: Which of the following is the best
Q74: What feature of a molecule is illustrated
Q76: Which of the following statements best describes
Q77: Which of the following wedge-dash drawings best
Q78: What does a ball-and-stick model show clearly
Q79: How many multiple bonds are in the
Q80: Which of the following models does NOT
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents