Which of the following figures best illustrates how two molecules of formaldehyde (CH2O) interact?
A) 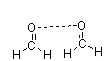
B) 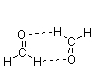
C) 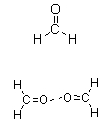
D) 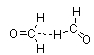
E) 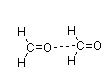
Correct Answer:
Verified
Q41: Which of the following molecules contains polar
Q54: Which of the following interactions is the
Q138: According to this diagram showing the boiling
Q140: What is the molecular shape of chloroform
Q141: What is the strongest type of intermolecular
Q143: What is the strongest type of intermolecular
Q144: Does ethanol have a permanent dipole?
Q145: Propane is a fuel commonly used in
Q146: Which of these molecules exhibits dipole-dipole forces
Q147: Which of the following molecules exhibits the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents