Does propane or octane (C8H18) exhibit stronger dispersion forces? 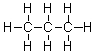
A) Propane, because the smaller molecules can get closer to one another
B) Propane, because propane has a larger permanent dipole
C) Octane, because octane has a larger permanent dipole
D) Octane, because octane has more surface area
E) They both have the same amount of forces
Correct Answer:
Verified
Q145: Propane is a fuel commonly used in
Q146: Which of these molecules exhibits dipole-dipole forces
Q147: Which of the following molecules exhibits the
Q148: What is the molecular shape of the
Q149: What is the electronic geometry of the
Q151: Which of the molecules do not have
Q152: What is the molecular shape of the
Q153: Ethanol is an alcohol found in wine
Q154: How does estradiol stimulate the growth of
Q155: Which of the following figures best illustrates
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents