What is the strongest type of intermolecular force that a caffeine molecule could form with other caffeine molecules? 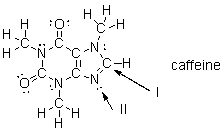
A) dispersion forces
B) nonpolar covalent bonds
C) hydrogen bonds
D) covalent bonds
E) dipole-dipole interactions
Correct Answer:
Verified
Q35: Tamoxifen has some key similarities to estradiol.Which
Q67: Antiestrogens are one type of molecule that
Q153: Ethanol is an alcohol found in wine
Q154: How does estradiol stimulate the growth of
Q155: Which of the following figures best illustrates
Q156: Which of the molecules below exhibits hydrogen-bonding
Q157: Which of the following best describes your
Q159: Which of the molecules exhibits the strongest
Q160: Which bonds in caffeine are polar covalent
Q163: Which of the following is a challenge
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents